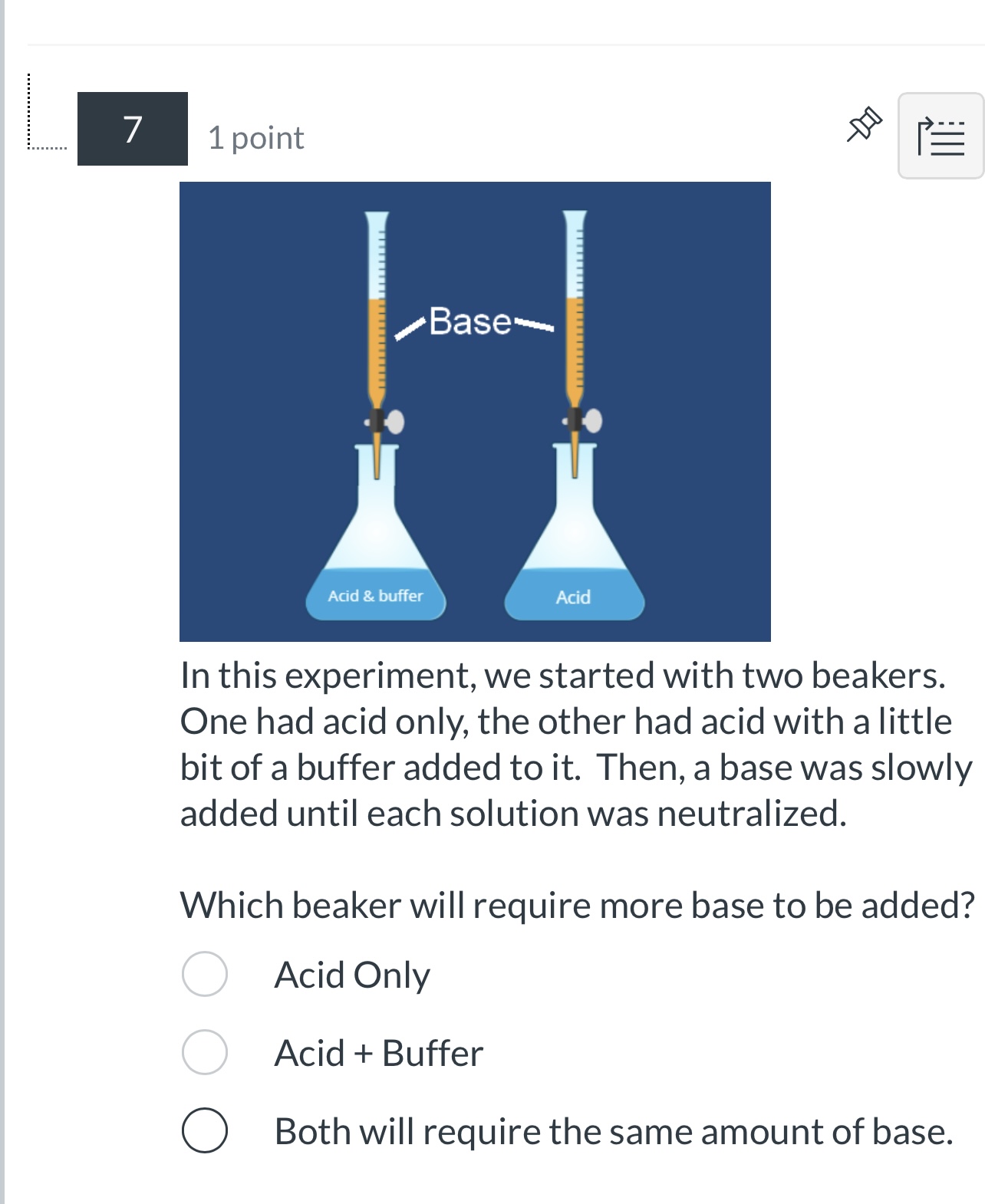
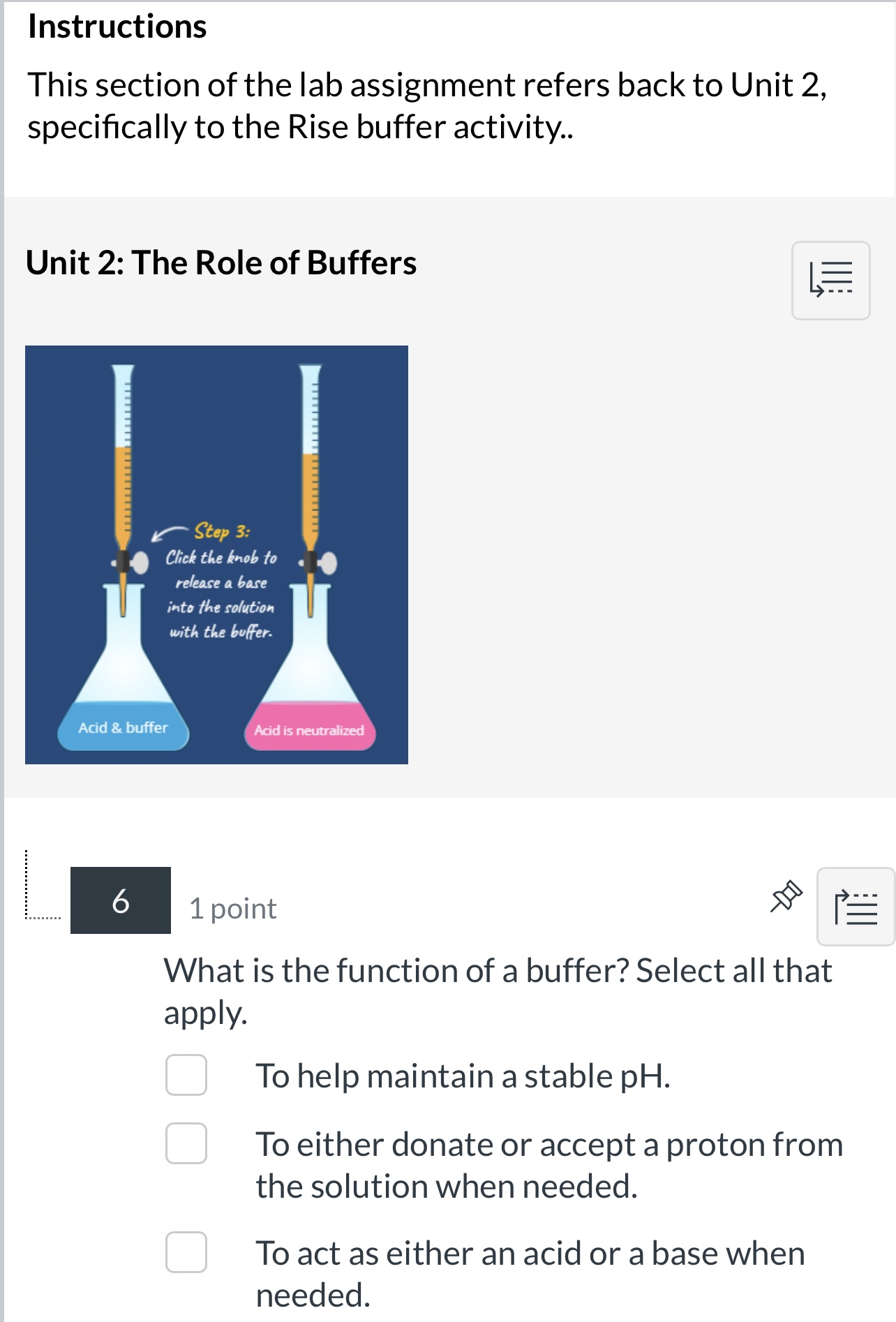
Imagine a chemical tug-of-war, a constant push and pull between acidic and basic forces. Neutralization is the truce, the equilibrium where these opposing forces reach a balance. But what exactly is a neutralized solution? It's not just a theoretical concept; it's a fundamental process underpinning everything from the environment to our own bodies.
In the simplest terms, a neutralized solution is what you get when you combine an acid and a base in just the right proportions. This reaction produces a salt and water, effectively canceling out the corrosive properties of both the acid and the base. The resulting solution is neither acidic nor basic, but sits comfortably in the neutral zone, typically with a pH of around 7.
Understanding the concept of neutralization goes beyond simple chemical equations. It's about recognizing the delicate balance that exists in nature and how disrupting this balance can have far-reaching consequences. Think of acid rain, a devastating example of what happens when excess acidity isn't neutralized. Or consider the importance of balanced pH levels in our blood, a crucial factor for maintaining healthy bodily functions.
The history of understanding neutralization is intertwined with the development of chemistry itself. Early alchemists, while pursuing more mystical goals, laid the groundwork for our understanding of acids and bases. Later, scientists like Svante Arrhenius and Johannes Brønsted refined these concepts, leading to the modern definition of neutralization as a proton transfer reaction between an acid and a base.
The importance of neutralization reactions stretches across a vast spectrum of applications. In industrial settings, it’s crucial for wastewater treatment, preventing environmental damage. In medicine, antacids rely on neutralization to relieve heartburn by counteracting excess stomach acid. Even in cooking, baking soda (a base) is used to neutralize the acidity of ingredients like lemon juice or vinegar.
A simple example of neutralization is the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH). These react to form sodium chloride (NaCl, table salt) and water (H2O). This seemingly simple reaction is a powerful demonstration of how neutralization transforms potentially harmful substances into benign ones.
Neutralization offers numerous benefits. It allows for the safe disposal of acidic and basic waste, protecting the environment. It plays a vital role in regulating pH levels in various contexts, from industrial processes to biological systems. And it forms the basis for many everyday products and applications, from cleaning agents to medications.
To effectively perform neutralization, you'll typically need an indicator to determine the pH of the solution. Slowly add the base to the acid (or vice versa) while monitoring the pH. Once the pH reaches 7, the solution is neutralized.
Advantages and Disadvantages of Neutralization
| Advantages | Disadvantages |
|---|---|
| Environmental protection through waste treatment | Potential hazards if neutralization is not performed carefully |
| Regulation of pH in various systems | Production of byproducts that may require further handling |
| Wide range of applications in different fields | Can be energy intensive in some industrial processes |
Five best practices for neutralization include: always add the base to the acid slowly, wear appropriate safety gear, use a suitable indicator, ensure proper ventilation, and dispose of neutralized solutions responsibly.
Real-world examples include treating industrial wastewater, using antacids for heartburn relief, adjusting soil pH in agriculture, neutralizing acidic spills in laboratories, and using baking soda in cooking.
Challenges in neutralization can include difficulty in accurately determining the endpoint, dealing with highly concentrated acids or bases, and managing the heat generated during the reaction. Solutions involve using precise equipment, implementing proper safety protocols, and utilizing cooling systems.
FAQs include: What is pH?, What is an indicator?, What is the difference between an acid and a base?, How do I determine the right proportions for neutralization?, What are some common examples of neutralization reactions?, Is a neutralized solution always safe?, How do I dispose of a neutralized solution?, What are the safety precautions for neutralization reactions?
One helpful tip is to always add the base to the acid slowly to avoid a rapid temperature increase. Another trick is to use a magnetic stirrer to ensure thorough mixing during the reaction.
In conclusion, understanding what is a neutralized solution is crucial for appreciating the delicate balance that governs many chemical processes in our world. From environmental protection to medical applications and even everyday cooking, neutralization plays a vital role. By grasping the fundamentals of this concept, we can better manage resources, develop innovative solutions, and safeguard our planet. Whether you’re a scientist, a student, or simply curious about the world around you, exploring the complexities of neutralization opens doors to a deeper understanding of chemistry and its impact on our lives. Continue your learning by exploring additional resources and experimenting with safe neutralization reactions under appropriate supervision. The world of chemistry is full of fascinating discoveries waiting to be unearthed, and neutralization is a key that unlocks many of its secrets.
Decoding the taper fade lifespan your ultimate guide
Arkansas food assistance navigating snap benefits
Understanding legal principles decoding apa arti asas hukum